Dec 7, 2020. By Kolemann Lutz

By applying microbes and iron to cleanse chlorinated chemicals from hazardous waste sites on Earth, we can improve the microbial and bioremediation properties and technologies associated with reducing perchlorates in Martian regolith.
Throughout the United States, hazardous waste sites continue to degrade human and environmental health. Superfund sites that manage waste sites are polluted with Trichloroethene (TCE) and Perchlorates (ClO4−), and other contaminants in surrounding surface and groundwater. In fact, close to every country has elevated levels of chloride (Cl-) and sulfate (SO4-) in surface and groundwater, which are primary criteria for ground water quality and assessment.
A new study published in July 2020 by the Union of Concerned Scientists found that more than 800 of the 1,000 hazardous Superfund sites on the U.S. East coast are at risk of flooding by 2040. Over 50 million Americans that live within three miles of one of these zones are at an increased risk for cancer and other serious diseases.
Commercially produced perchlorate salts are extensively used in the manufacture of solid rocket propellants, munitions, fireworks, airbag initiators for vehicles, matches, and signal flares. TCE is employed to degrease and cleanse metal parts in mechanical equipment. Due to the common use of the chlorinated ethenes at DoD-operated military sites, 90% of ClO4 is manufactured for the defense and aerospace industries, primarily in the form of inorganic ammonium perchlorate (NH4ClO4). TCE and ClO4- typically co-occur in soil and groundwater with high SO4 concentrations, particularly in the U.S. southeast. Perchlorates are an emerging toxicological chemical of concern that is widespread in drinking water aquifers, providing a primary pathway for human ingestion and exposure.
Perchlorates are considered toxic for people, but they don't necessarily pose a problem for some microbes. A wide variety of microorganisms can reduce ClO4 to O2 and chloride by respiration with available electron donors (e.g., H2, acetate), providing more energy for microorganisms. At many contaminated waste management sites, Dehalococcoides mccartyi bacteria are used to bioremediate trichloroethylene (TCE).
Researchers from Arizona State University's Biodesign Swette Center for Environmental Biotechnology conducted the first study investigating the use of zerovalent iron (Fe0 or ZVI) and microbial reduction of TCE and ClO4 to achieve enhanced anaerobic microbial activity and shorter bioremediation timeframes. As the most commonly used zerovalent metal for bioremediation, ZVI is used in filters, electrodes, and trenches to degrade or sequester contaminants in groundwater and soil.
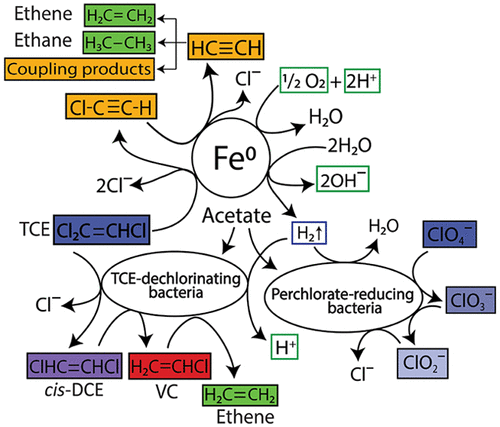
After sparging groundwater with nitrogen (N2), researchers added the microbe Dehalococcoides mccartyi to soil and groundwater samples from a contaminated Superfund site in Goodyear, Arizona with a groundwater ratio of 1:3. After microcosm bottles were sealed and shaken starting on day 14, dechlorinating enrichment cultures with non-bioaugmented (high Fe0) and biostimulated microcosms (low Fe) were studied across four 14-day semibatch experiment periods (56 days).
The oxidation of zero valent iron generates water-soluble Fe2+ ions that travel with the flow of groundwater and can reduce extra oxygen, yielding Fe3+. Ferrous iron (Fe2+) and ferric iron (Fe3+) can precipitate on microbial cell surfaces and be incorporated into the cell wall, impeding TCE dechlorinating and CLO4- reducing microorganisms.
Using a microcentrifuge, DNA extraction kit, qPCR, and high-throughput DNA sequencer, researchers sequenced microbiological biomass samples from microcosms at the beginning and end of each of the four semibatch cycles.
The study demonstrated that low concentrations of partially oxidized (aged) zerovalent iron and Dehalococcoides mccartyi bacteria showed synergistic abiotic and microbiological ClO4- reduction and TCE dechlorination into harmless chloride ions and ethene, also known as ethylene (C2H4).
ClO4- was completely reduced after 28 or 42 days in all microcosms with Fe+2, indicating less inhibitory effects of Fe+2 compared to Fe0 on perchlorate-reducing bacteria (PRB), which utilize ClO4- as a a terminal electron acceptor. However, high concentrations of Fe0 inhibited TCE and perchlorate reduction while ferrous iron (Fe2+), an oxidation product of Fe0, significantly slowed down TCE reduction.
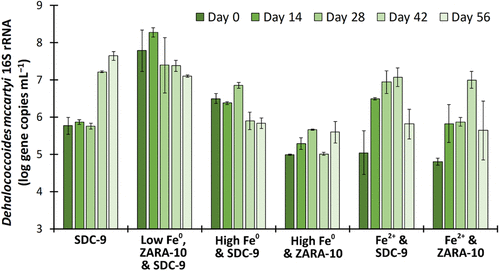
The concentration of Fe, availability of electron acceptors such as hydrogen, and initial microbial community play a major role in perchlorate reduction and in situ bioaugmentation.
Many terrestrial groundwater contaminated sites are abundant in SO4 (in quantities of 10 to 500 mg per liter), which is also an electron acceptor for sulfate-reducing bacteria. High sulfate concentrations over 500 mg L-1 can decrease the rates of reductive dechlorination in aquifers while high concentrations of sulfide tend to inhibit many microorganisms.
As the iron-enriched crust of Mars contains twice as much iron as found in Earth’s crust, high amounts of iron and sulfate pose challenges for cultivating crops in Martian soil. Considering the Martian surface material contains abundant quantities of Mg-, Ca-, and Fe+3 sulfates, high SO4 concentrations pose challenges for early microbial settlers, demonstrating a need to optimize the sulfate and perchlorate reducing bacteria life cycles and dual purpose microbial communities.
Because sulfate and sulfide inhibit many microbial functions, perchlorate reducing bacteria can be introduced and tested in Martian soil following the synergistic SO4 reduction or bioremediation to foster greater bacterial nutrient exchange for plants and to support Fe, Ca, and Mg mineral extraction.
Perchlorate reduction and management procedures are an essential component of early settlement plans. On the Martian frontier, perchlorates are widespread in the form of chlorinated hydrocarbons in soils at concentrations between .5 and 1%. Although ClO4- is widespread in Martian water ice, industrial-scale brine water electrolysis plants can extract the perchlorates from dirty water.
By expanding on the research and practice of perchlorate reduction in contaminated water, ground soil, and hazardous waste sites to improve the quality of life on Earth, humanity can simultaneously advance the practice and field on chlorine-abundant planets.
Research Paper. Srivatsan Mohana Rangan, et al. Synergistic Zerovalent Iron (Fe0) and Microbial Trichloroethene and Perchlorate Reductions Are Determined by the Concentration and Speciation of Fe. Environmental Science and Technology. Nov. 2, 2020.

